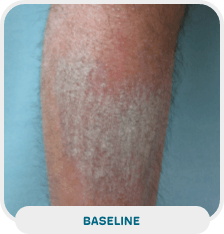
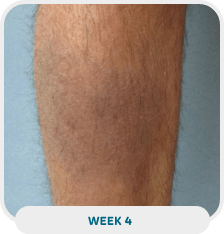
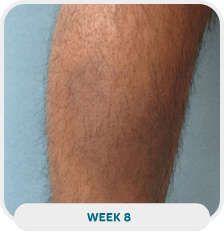
Patient: 38-year-old male
Initial exam: January 21, 2022
Affected area: Lower leg
Treatment approach:
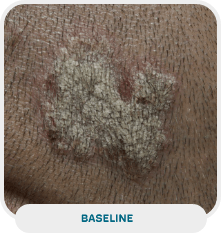
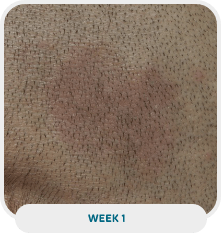
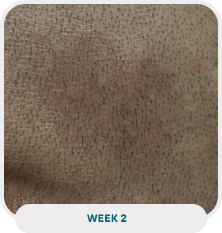
Patient: 51-year-old male
Initial exam: 2015
Affected area: Scalp, back, and extremities (upper and lower)
Current exam:
Treatment approach:

INDICATION: Wynzora® (calcipotriene and betamethasone dipropionate) Cream, 0.005%/0.064% is indicated for the topical treatment of plaque psoriasis in patients 18 years of age and older. ADVERSE EVENTS: In the pivotal trial, the most common adverse reactions (≥1%) were: upper respiratory infection (7%), headache (2%), and application site infection (1%). WARNINGS AND PRECAUTIONS: For topical use only. Patients should not use more than 100g of Wynzora® Cream per week. Do not use near or in the mouth, eyes or intravaginally. Avoid using Wynzora® Cream on the face, groin or armpits, or if they have atrophy at the treatment site. Discontinue use once plaque psoriasis is under control or at 8 weeks. Do not use Wynzora® Cream with occlusive dressings. Hypercalcemia and hypercalciuria have been observed with topical use of calcipotriene. Wynzora® Cream can cause reversible HPA axis suppression with the potential for clinical glucocorticosteroid insufficiency during and after withdrawal of treatment. Wynzora® Cream may cause vision problems, including increasing the risk of cataracts and glaucoma. It is not known if Wynzora® Cream may harm your unborn baby. Breastfeeding women should not apply Wynzora® Cream directly to the nipple or areola; it is not known whether topically administered calcipotriene and betamethasone dipropionate is absorbed in human milk.
Please see Full Prescribing Information for Wynzora.
You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Reference: 1. Data on File. MC2 Therapeutics.
